Abstract
Background
It is reasonable to incorporate quality of life (QoL) assessment into the comprehensive evaluation of treatment outcomes in chronic myeloid leukemia (CML) patients (pts) with deep molecular response (DMR) who enter the treatment-free remission (TFR) phase and stop therapy by tyrosine kinase inhibitors (TKIs). QoL assessment was included into the design of Russian prospective multicenter trial RU-SKI along with the clinical outcomes evaluation.
Aim
To study QoL in chronic phase (CP) CML pts with DMR before stopping TKI treatment and during TFR observation.
Materials and methods
The study has been conducted within the clinical approbation supported by the Ministry of Health of RF. The CML CP pts with therapy by any TKI ≥ 3 years (yrs) and stable DMR (BCR-ABL ≤0.01% IS) during ≥2 yrs were enrolled. Pts who met these criteria and had previous resistance to any TKI were also eligible. TKIs were resumed in case of major molecular response loss (MMR, BCR-ABL>0,1%). The QoL questionnaires RAND SF-36 and EORTC QLQ C30 were filled out by the pts before stopping TKI treatment and at 1, 3, 6 and 12 months (mo) after treatment discontinuation. The comparison group consisted of healthy persons matched by age and gender to CML CP pts (n=97). The Mann-Whitney test, paired Wilcoxon test and Generalized Estimation Equations (GEE) with adjustment to age, gender, the risk group according to Sokal score and duration of TKI treatment were used for the statistical analysis.
Results
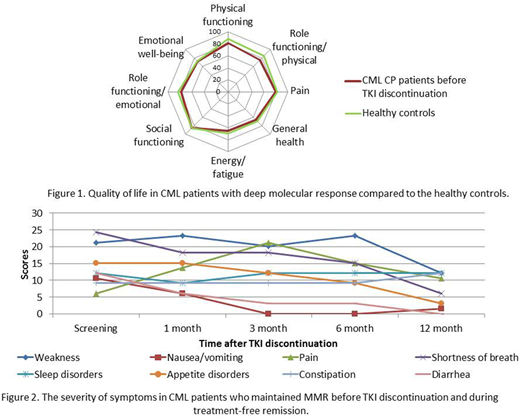
QoL assessment was performed in all 99 CML CP pts who were enrolled into the trial during a period from Aug 2015 till Dec 2017. The TKIs before treatment cessation were as follows: imatinib and second-generation (2G) TKIs were used in 69(70%) and 30(30%) pts accordingly. 2G TKIs were used in 9(30%) and in 21(70%) pts as 1st and 2nd line accordingly. Mean age was 47±14.5 yrs, 48.5% were males, 12.1% had high Sokal risk score. The physical functioning of CML pts before stopping TKI treatment compared to the healthy controls was significantly worse (p<0.05). Other QoL scales before stopping TKI treatment were slightly worse with no statistically significant difference compared to healthy controls (figure 1). MMR was lost in 45 pts, and was maintained in 54 pts, Me follow-up time after TKI stop was 14,9 mo (range 2,5-32). The significant improvement of role physical functioning and role emotional functioning was found (p<0.05) in pts who maintained stable MMR during 12 mo after TKI discontinuation. The Integral QoL Index increased from 0,58 to 0,72 (p<0,001) in these pts. The decreasing of nausea/vomiting, diarrhea, shortness of breath at different time points (p<0.05) during TFR was revealed (figure 2). The QoL and symptoms before TKI interruption and after reinitiating of TKIs in 45 pts who resumed treatment were similar. The Integral QoL Index before and after stopping TKI treatment was 0.62 vs 0.64 (p>0.05).
Conclusion
The QoL in CML CP pts with DMR on TKI treatment is slightly worse and physical functioning is significantly lower as compared to healthy controls. The positive changes of the role functioning along with TKI treatment-related symptoms decrease were revealed in pts who maintained stable MMR during 12 mo of TFR. No changes in QoL and no new symptoms were found in pts who reinitiated treatment before and after TKIs interruption. The results of QoL assessment may contribute to optimization of the treatment strategies of CML patients with DMR.
Chelysheva:Fusion Pharma: Other: provided consultations ; Bristol Myers Squibb: Other: provided consultations and performed lectures; Novartis: Other: provided consultations and performed lectures. Shukhov:Bristol Myers Squibb: Other: provided consultations and performed lectures ; Novartis: Other: provided consultations and performed lectures . Turkina:Novartis: Other: provided consultations; Bristol Myers Squibb: Other: provided consultations; Phizer: Other: provided consultations; Fusion Pharma: Other: provided consultations. Ionova:Takeda: Research Funding; BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal